What Are PFAS?
PFAS are a group of chemicals that have been manufactured since the 1940s for use as ingredients in a wide variety of everyday products, from clothes and firefighting foams to food packaging and cookware.
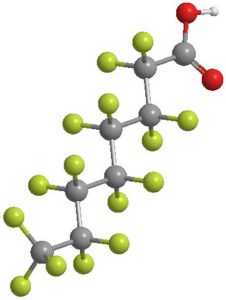
Thousands of different PFAS exist, but they all share an important quality: many PFAS are long-lasting and do not degrade easily, which allows them to bioaccumulate and persist in the environment. This has led to some scientists calling them “forever chemicals.”
PFAS is an acronym for per- and polyfluoroalkyl substances, a class of chemical compounds championed for their non-stick properties. PFAS molecules contain a chain of carbon and fluorine atoms, which are strongly bound together. These strong carbon-fluorine bonds make the chemicals difficult to degrade.
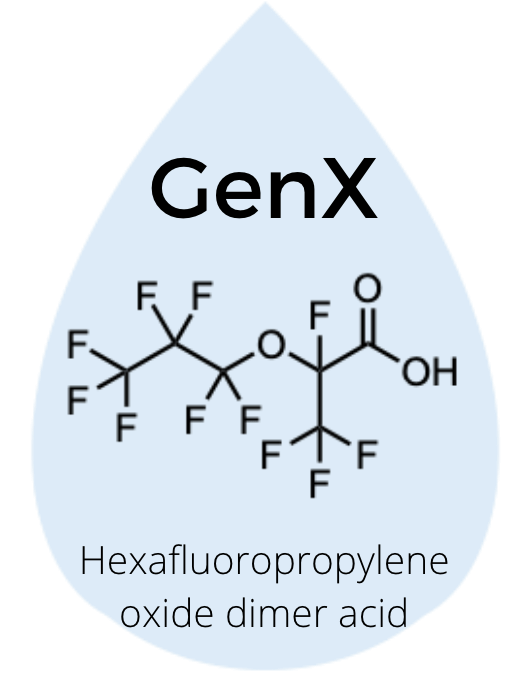
Some PFAS have been studied more than others, such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), which are found in a wide variety of locations. Another well-known PFAS is GenX, which is a trade name for two chemicals – hexafluoropropylene oxide dimer acid and its ammonium salt – that are produced as a byproduct of certain processes such as manufacturing non-stick coatings.


Resources
- Environmental Working Group’s interactive map of PFAS contamination
- Environmental Working Group’s PFAS news and research
- Environmental Protection Agency’s current understanding of PFAS.
- Environmental Protection Agency’s recommendations for limiting PFAS exposure
