Sorbents to be Tested
New testing and remediation pathways for the removal of PFAS from water is of the upmost importance. PFAS have been detected in numerous water sources throughout North Carolina. The ultimate goal is to assess performance of a range of technologies in different water matrices. Testing will include Novel Sorbents, as well as leading sorbent technologies such as granular activated carbon and anion exchange resins. By studying PFAS removal by leading and emerging technologies in waters from across the state, this study is expected to help inform treatment technology selection for water systems throughout North Carolina.
Granular Activated Carbon (GAC) and Anion Exchange Resins (AER): Sorption technologies using granular sorbents (i.e., GAC and AER) are state-of-the-art processes for PFAS removal. Commercially available GAC and AER have been implemented across the nation for PFAS removal. The two most important factors controlling PFAS sorption of granular sorbents are PFAS carbon chain length and functional group; long-chain PFAS are more amenable to GAC and AER sorption, whereas PFAS with sulfonate groups in their structure are generally removed to a greater extent than PFAS with carboxylate groups. Studies have also shown that sorbent pore structure plays a role in PFAS removal, and AER are generally more effective than GAC at removing PFAS. The contrast in performance between GAC and AER can be partially explained by the differences in their respective dominant sorption mechanisms: hydrophobic interactions for GAC and electrostatic interactions for AER.
Limitations of GAC and AER: A primary challenge for both GAC and AER is that they suffer from low removal efficacy of short-chain PFAS. GAC and AER remove contaminants non-specifically resulting in saturation contributed by non-fluorinated species because competing non-fluorinated species (i.e., natural organic matter, anionic salts) are present at much higher concentrations than PFAS. Limited specificity for PFAS removal means that GAC and AER must be replaced more frequently, increasing treatment cost for water systems, and ultimately utility ratepayers across the state.


Ionic Fluorogels (IF): To address the need for granular sorbents with enhanced PFAS selectivity, we recently formulated a platform approach for developing novel sorbents that remove PFAS selectively over competing background contaminants.1 and 2 We refer to this novel class of sorbents as Ionic Fluorogels (IF). Our results show that IFs outperform commercial sorbents in North Carolina Waters for a broad range of PFAS (i.e., greater capacity for PFAS removal, especially for short-chain PFAS, and longer run times without observing PFAS in the treated waters). Many of the novel sorbents we have developed are not fluorinated, however, IF are a useful “proof-of-concept” that demonstrate the power of using contemporary chemistry and engineering to solve challenging problems in water remediation.
Ionic Fluorogels Compared to State-of-the-Art Technologies
Many of the novel sorbents we have developed are not fluorinated. However, these published data on IFs demonstrate the performance of selective PFAS remediation materials relative to commercial sorbents.
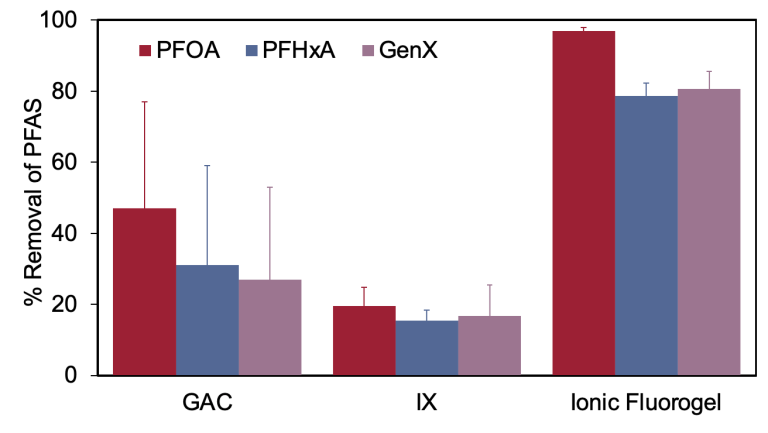
Ionic Fluorogel was tested alongside commercially available GAC and IX technologies for the percent removal of three different PFAS. PFOA, PHxA and GenX were selected due to their differing chemical structures which affects sorption.
The high percent removal rate from ionic fluorogels demonstrates its high selectivity and efficacy for removing PFAS in comparison to state-of-the-art alternatives.
Ionic Fluorogel Percent Removal with 21 PFAS
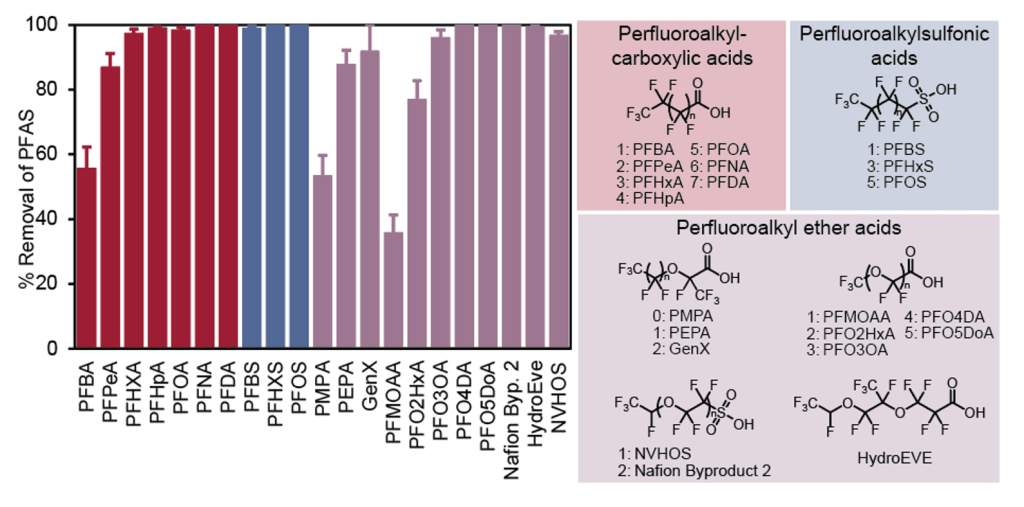 Percent removal of PFAS varies depending on the class of PFAS. The chemical structure and its properties affect the efficacy of sorbents, including Ionic Fluorogels, in removing PFAS. The above graph demonstrates the Ionic Fluorogel’s percent removal in batch tests for 21 different PFAS that primarily belong to three classes of PFAS.
Percent removal of PFAS varies depending on the class of PFAS. The chemical structure and its properties affect the efficacy of sorbents, including Ionic Fluorogels, in removing PFAS. The above graph demonstrates the Ionic Fluorogel’s percent removal in batch tests for 21 different PFAS that primarily belong to three classes of PFAS.
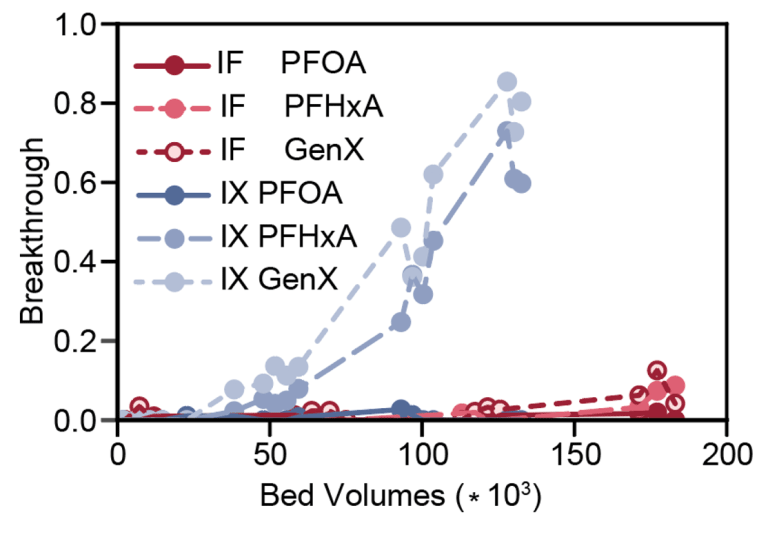 Breakthrough is evaluated as a function of bed volumes which is the measure of how much water has been filtered by the technology. The Ionic Fluorogel was tested alongside commercial AER with three types of PFAS. Results show that breakthrough of short-chain PFAS (i.e., GenX and PFHxA) occurred at least three times earlier for ion exchange resins than for the Ionic Fluorogel. This indicates that in a filter system, three times as much water can be treated by the Ionic Fluorogel than by the ion exchange resin before observing short-chain PFAS in the filtered water.
Breakthrough is evaluated as a function of bed volumes which is the measure of how much water has been filtered by the technology. The Ionic Fluorogel was tested alongside commercial AER with three types of PFAS. Results show that breakthrough of short-chain PFAS (i.e., GenX and PFHxA) occurred at least three times earlier for ion exchange resins than for the Ionic Fluorogel. This indicates that in a filter system, three times as much water can be treated by the Ionic Fluorogel than by the ion exchange resin before observing short-chain PFAS in the filtered water.
